NIRS-EEG protocol E.Noordanus: Difference between revisions
| Line 341: | Line 341: | ||
===Measurement preparation=== | ===Measurement preparation=== | ||
[[File: EEG_NIRS_preparation.png|thumb|EEG preparation]] | [[File: EEG_NIRS_preparation.png|thumb|EEG preparation]] | ||
====Needed for skin/head cleaning for EEG preparation (see Figure 23: EEG preparation)==== | |||
* Disinfectant alcohol for cleaning of the forehead, the mastoid bones, below the eye, next to the eye where the electrodes to measure blinking will be connected. Use this also to clean the scalp (first check with the subject). | * Disinfectant alcohol for cleaning of the forehead, the mastoid bones, below the eye, next to the eye where the electrodes to measure blinking will be connected. Use this also to clean the scalp (first check with the subject). | ||
* Remark: when the subject used gel or other hair products except shampoo in the hair, the hair needs to be washed first. | * Remark: when the subject used gel or other hair products except shampoo in the hair, the hair needs to be washed first. | ||
Revision as of 12:13, 10 January 2024
How to use this protocol
This protocol describes the procedures, equipment and software used in the NIRS-EEG lab. The following reading is recommended.
- All readers: read chapter 2.
- For readers who use existing, well defined measurement procedures and do not need to make any changes to the software or hardware setup: read chapters 3 and 4. In addition, for readers who perform EEG measurements: browse through chapter 9 and read chapter 10 thoroughly; for NIRS users: browse through chapter 11 and read chapter 12 thoroughly.
- For readers who want to change an existing Matlab script and/or RPvdsEx circuit or create a new experiment: read chapters 3, 4, 5, 7, and 8. In case of big changes, it is advisable to read chapter 6 in addition and contact a senior researcher.
- For readers who want to look in detail at EEG measurements: read chapters 9 and 10.
- For readers who want to look in detail at NIRS measurements: read chapters 11 and 12.
- For information concerning the TDT equipment and all connections to the TDT equipment: read chapter 6.
First user


Lab procedures
- Covid-19 prevention lab rules: COVID-19
- Safety first!! Please, keep your own safety and that of your subjects always in mind. Number - who to call in case of emergency:
- When handling equipment always take care! In case you are new to the lab contact a senior researcher for help and also when you are unsure on how to handle equipment!
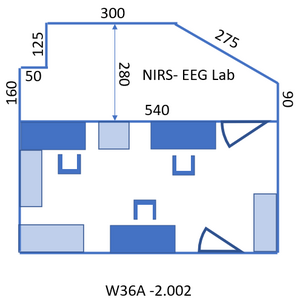
- No eating and drinking allowed in the lab. This includes drinking of water. Exception: during an EEG measurement drinking from water bottles that can be closed is allowed for subject and experimenter in the experimental room (see Figure 3), due to the long procedure of fitting the cap and the importance of keeping both subject and experimenter awake. Take care not to spill any water!
- Being in the lab before 7 and after 18 o’clock and on weekends is not preferred. The entrance to the lab (the big doors after the elevator) will be locked from 18 o’clock to 7 o’clock the next day! Make sure you always leave the lab before 18 o’clock! In case of entering before 7 o’clock and staying after 18 o’clock a Radboud card with access rights (with pin code - arrange via Judith) is necessary. When you are planning to be in the lab outside these times, especially for weekends, authorization of a senior researcher is required.
- Report to your senior researcher if supplies are missing and if anything is broken or doesn’t function. In the latter case also report this in the Issue tracking system at gitlab.science.ru.nl.
- Depending on the current Covid-19 regulations of the Radboud university, Donders center and the government, the following rules will apply. The distance between the experimenter and the subject will be at least 1.5 meter, except during the placement of the EEG cap, when the experimenter will wear a disposable mouth-mask, a clean lab coat, and gloves. The subject can also wear a mouth-mask and gloves (available in the lab), but that is not required. All equipment that needs to be touched by the subject will be cleaned and disinfected beforehand and between measurements. The airco must be on and before and after the measurements the doors will be kept open until you leave.
- Always keep record of your experiments in your lab journal.
Handling subjects
- Each experiment must be recorded in your lab journal (blue book),
- Each subject needs to sign the Consent form (located in the cabinet on the left side of the lab, to the left just out of the view shown in Figure 2) before start of the experiment.
- The questionnaire (also in above mentioned cabinet), must be completed for each subject and afterwards pasted into your lab journal.
- Add ?: Ear checking/cleaning procedure?
- Add: always start with audiogram measurement.
- Add: Cleaning procedure for this lab (it is now and then necessary to clean the floor etc…) + where to empty the bins.
Cleaning procedures
Washing of towels and lab coats is arranged in the Covid-19 procedures.
Procedure for data storage
Important: follow the RDM (Research Data Management) procedure of the Donders, see: https://www.ru.nl/donders/research/research-data-management/ Put your data (and only your data, no scripts) in directory: C:\DATA\<folder with your initials> or in the my documents directory of the PC you use for the measurement. Backup your data in the Donders repository (data.donders.ru.nl) or to one of the department network drives. You can use the network location: mbaudit3-srv.science.ru.nl for EEG data and mbaudit4-srv.science.ru.nl for fNIRS data. Of these network drives a backup is made regularly. On the lab PC: click the Start button, click “Computer” => Map network drive. Choose a drive letter and fill in for the Folder: “\\mbaudit6-srv.science.ru.nl\mbaudit6”. Check the options “Reconnect at logon” and “Connect using different credentials”. Use your science login to connect.
- These network drives are intended to back up your raw data. You may copy data to your own PC (or external drive), but you should never modify or delete the files on the network drives! For more info, see Research Data Management.
- Also save a backup of all scripts and other files that are important to repeat the experiment and your analysis on the network drive.
When you don’t need the data any more on the measurement PC and you entered the data in the Donders repository, you can remove the data from the measurement PC.
Lab reservation
Reserve lab time with qreserve: [1](https://www.qreserve.com). First ask for a new account or – in case of students – for the login of your supervisor. Click “Department of Biophysics” on the Dashboard page and choose Resources - NIRS-EEG Lab.
Location
Access to the lab: take the last elevator at the opposite end of the building from wing 8 to floor -2 and follow the signs “Biophysics labs” to the room: A1 -2.002, the NIRS- EEG lab. Entry code of the room: ask your supervisor. To walk back to the elevator: follow the signs “Huygensgebouw”. Toilet: exit the lab to the right, turn left after the graffiti, the two doors to the right after the stairs and before the big door. Figure 1 gives the layout of the lab. The lab consists of two rooms, see Figure 2 and Figure 3. The first, where you enter the lab, will be referred to in this protocol as “control room” since the PC’s are located over there. The second will be called “measurement room”.
Setup of the main PC

The PC to the left in the control room is used to generate stimuli and control the experimental flow over the TDT (Trucker-Davis Technologies, USA, see next chapter).
- Press the on button of the PC (see Figure 4).
- Ask your supervisor to create an account on the PC you use or provide you with the login information of an existing account. In the latter case, you don’t have to perform the steps below, but read them so you know where to find the scripts and the information you need. Remark: references in this protocol to “your initials” or “your first name” indicate the name of this user account.
- Experimental and data analysis scripts can be found at: https://gitlab.science.ru.nl (more specifically: https://gitlab.science.ru.nl/marcw/biofysica/tree/master). Ask Marc van Wanrooij to give you access to biofysica and biophysics_labs. Here you can:
- Report / view Issues in https://gitlab.science.ru.nl/marcw/sphere_lab/issues. Label your issue with [EEG/NIRS]
- Download the most recent version of all software and put that in C:\gitlab\[Folder with your first name]\biofysica.
- To easily add all files in the biofysica directory to your Matlab path, add a file “startup.m” in the directory C:\users\<your user name>\My Documents\MATLAB with the line:
addpath(genpath('C:\gitlab\<Folder with your first name>\biofysica')); - Never change the files in C:\gitlab\<Folder with your first name>\biofysica, so you can easily update all files from gitlab. Updating ‘the git way’ instead of plain downloading requires a different approach, using git tools. This may not be needed by all users, but if you change programs or scripts frequently, using git tools is very convenient.
- Place your own scripts (Matlab and rcx files, see chapter: “Create stimuli and interact with the buttonbox”) in (a subdirectory of) C:\users\<your user name>\My Documents\MATLAB. Be sure to always use new names for your own scripts and functions to avoid mix-up with the files in the biofysica directory.
- See “$2.1 Lab procedures” above for data storage and backup procedures.
3. Overview of the Measurement Setup

Tucker-Davis Technology
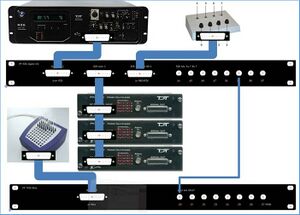
The TDT equipment (see Figure 5) is used to generate stimuli and present them on the speakers (or a headphone) in the measurement room. The RZ6 is central: it generates the stimuli and presents them to the PM2R multiplexers (labeled: “device 1”, “device 2”, and “device 3”), which send the stimuli to the correct speaker(s). Furthermore, the RZ6 registers the output of the button box (which button is pressed by the subject) and controls the input (which lights turn on) to the button box. It can do (limited) data acquisition and processing. The RZ6 also sends timing signals (“triggers”) to the EEG measurement equipment (the Refa; TMSi) in the measurement room. That is the only connection between the TDT and the EEG equipment.
Table 1 gives an overview of the coding to activate each of the 32 speakers. Negative angles represent the left hemi field (left of the center). Device and channel counting start with 0.
| Speaker position (degree) | Device | Channel | Speaker position (degree) | Device | Channel |
|---|---|---|---|---|---|
| 0 | 0 | 0 | |||
| -5 | 2 | 1 | 5 | 0 | 1 |
| -10 | 2 | 2 | 10 | 0 | 2 |
| -15 | 2 | 3 | 15 | 0 | 3 |
| -20 | 2 | 4 | 20 | 0 | 4 |
| -25 | 2 | 5 | 25 | 0 | 5 |
| -30 | 2 | 6 | 30 | 0 | 6 |
| -35 | 2 | 7 | 35 | 0 | 7 |
| -40 | 2 | 8 | 40 | 0 | 8 |
| -45 | 2 | 9 | 45 | 0 | 9 |
| -50 | 2 | 10 | 50 | 0 | 10 |
| -55 | 2 | 11 | 55 | 0 | 11 |
| -60 | 2 | 12 | 60 | 0 | 12 |
| -70 | 2 | 13 | 70 | 0 | 13 |
| -80 | 2 | 14 | 80 | 0 | 14 |
| -90 | 2 | 15 | 90 | 0 | 15 |
Table1: device (multiplexer number) and channel coding for each speaker.
EEG system (Refa)
The EEG system (Refa) has 72 channels. It is connected via an orange bi-directional optical fiber to the PC to the right of the TDT in the control room. The EEG data is measured and saved continuously during the experiment (so not per trial). In a separate channel (channel 73, named Digi) the trigger signals from the RZ6 are saved. Only the button response and the timing of the button press need to be saved via the RZ6 and the Matlab script (of course you are free to program a script that saves all kind of other information, for example regarding the exact timing of the stimuli).
4. Present stimuli
RZ6


- Check if the connection of the cables is correct for your experiment.
- Attention: always leave the attenuation knob (to the left in Figure 6) at 0.
- If the electrostatic speaker driver is not being used, make sure that the ON/OFF switch (to the right in Figure 6) is in the OFF position to reduce noise on the RZ6.
- To start the TDT: switch the four power buttons indicated in Figure 7.
- Do a test run with your Matlab script.
- When you want to hear the A or the B sound output directly from the small speaker integrated in the RZ6 (lower right corner in Figure 6), you can put the switch next to the speaker either to “A” or “B”. When you hear nothing turn the volume up with the knob (in the middle row) labeled “Mon level”.
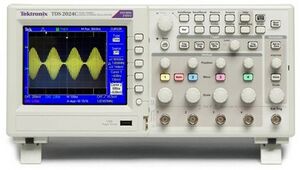
- Connect the analog output signal (for example output OUT-A) to one of the inputs of the oscilloscope – see Figure 8 – to check the output signal.
- If everything is prepared do a test run with your subject before starting the real measurement.
- For performing EEG measurements see chapter 10. For NIRS measurements: see chapter 12.
Oscilloscope
The large round button in the right lower corner changes the x-axis: the time per division (shown in white in the center below the x-axis). There are four input channels, marked 1 to 4 with different colors. You can change general channel settings by pressing the colored button of the channel. Each bigger knob below the number changes the y-axis of the channel: the voltage per division (shown in the color of the channel below the x-axis). Each smaller knob above changes the offset of the y-axis of that channel. The “Probe ..X Voltage”, listed in the display to the right, should be at 1X voltage. Manual: http://docs-europe.electrocomponents.com/webdocs/07dd/0900766b807ddf1d.pdf.
NIRS equipment
%todo
Create Stimuli and Interact with the Button Box
All information regarding TDT system 3 equipment (see Figure 5) can be found in the directory TDT of the gitlab\<Folder with your first name>\biofysica directory that you created in chapter 0. You can also find information in directory C:\TDT. Links for up-to-date manuals on the Internet:
When you want to change a circuit, the RPvdsEx manual is important. In the next chapter, the hardware setup is described. Ruurd Lof made a toolbox with standard functions that you can use, for info ask him or Günter Windau.
Examples of EEG measurement implementations, consisting of an RPvdsEx circuit file (extension: .rcx) that can be run out of Matlab, a Matlab file (.m) to run the application, and possibly files with the experimental settings (.mat), can be found in gitlab\<Folder with your first name>\biofysica\eeg. If there is a circuit file with a name ending on “_standalone”, you can load and run this in RPvdsEx (first turn on the TDT equipment), further see chapter 0 and play with the settings.
Always first turn on the TDT equipment (see Figure 7) and then run the Matlab file.
Sound hardware
RZ6 sound processor
Info about the RZ6 can be found here: RZ6 info page.
Figure 9 shows the devices, patch panels, and connections on a photograph of a part of the TDT rack.

==Hardware depicted in figure 10== 1: PM2Relay multiplexer device 0 2: PM2Relay multiplexer device 1 3: PM2Relay multiplexer device 2 4: Analog out A of the RZ6 5: Analog out B of the RZ6 6: Analog out A and B combined for headphone 7: Digital I/O of the RZ6 8: Patch panel that redistributes the Digital I/O of the RZ6 (nr 7), labeled “Bytes A, B,C” to different connectors: -byte C, input for the multiplexers, to the connector labeled “RZ6 byte C”; -I/O for the response box via “RZ6 bits A0…3 B0…3” -output for triggers via the BNC’s A4…A7 (byte A bit 4-7) and B4…B7 (byte B bit 4-7). 9: Patch panel that sends the input to the BNC connectors D0…D7 to the Refa 8-bit trigger input (marked 3 in Figure 10). 10: Flatcable connecting the Digital I/O of the RZ6 to the RZ6 Digital-I/O patch panel (8). 11: Flatcables connecting the PM2Relay multiplexers 12: Monitor speaker (for example to connect a microphone from the lab) 13: Medusa (has been removed) 14: Analog input for the multiplexers (connect with 4 or 5)
The upper right corner of Figure 10 (see also Figure 6) shows the Input/Output interface of the RZ6, the right part of the RZ6. The upper row contains the input connectors, to the left for example you can connect a microphone. The frequency response of the analog inputs is DC-0.44*Fs if Gain=1. With amplification the inputs are AC coupled to prevent clipping. An external anti-aliasing filtering is not needed because sigma-delta ADCs are used. The middle row contains the analog output.
Please leave the left knob for manual attenuation (to the left in Figure 6) on 0. If you want to attenuate the output you can use the attenuation task in BIOX. Out-A and Out-B are the output of the A and B channels, the signals for the speakers. The voltage out is +/- 10 Volt (stay below the 10V to avoid clipping), see the “RZ6 Multi I/O Technical Specifications” part of the RZ6 spec. These signals are also passed to two stereo headphone outputs, the connector labeled “A&B” and “Mon”. An extension cord for a headphone is fed through the wall to the measurement room. Use the “Mon” output for monitoring only. The analog outputs are amplified and can deliver up to 12 Watt RMS into 4Ω loads. BNC and A&B jacks have separate power amplifiers.
BIOX
%todo
Calibration
Todo: Protocols for Calibrations
- Calibration of speakers (sound levels + faithful reproduction of the frequencies / waveform). The sound level meter is used for measuring the output of the speakers. Measurement results are stored in the grey box in the cabinet to the left of the control room.
- Calibration of the sound level of an insert earphone, see: mbaudit6: Description_insert_ear_calibration_181115.docx
- Record the output of an insert earphone.
- Distance measurements
- Measurement of the angle between speaker and subject
- Check if the surface of the speaker is sufficiently perpendicular to the line from the center of the speaker to the head of the subject.
EEG equipment
%todo
Manuals
- To do: checken
- SAGA manual and technical specification of the SAGA can be downloaded via: TMSI knowledge base
- Manuals for water and gel electrode caps %todo
- Manual for the TMSi Polybench software: open the patient data manager on the EEG PC, now you can access it through the question mark in the upper right corner. Further a description and reference can be found at http://www.appliedbiosignals.com/index.php?id=polybench. Polybench is a software application toolbox for the creation of customized applications that work with (all kind of) physiological signals. You can analyze and process signals, also real-time. Further you can design protocols and graphical user interfaces. The Quick Recording application with the Polybench configuration for the Refa can be downloaded from %todo.
- Matlab class to import Refa measurements into Matlab or to perform the measurement directly from Matlab without using the above mentioned Polybench software can be downloaded from TSMI Software.
The Refa

According the Refa manual (see Manuals) no high pass or low pass filters that can cause signal phase shifts or filter overflows are present in the Refa. The following filtering is applied in the Refa: a 1st order low pass filter before the analog-to-digital converter (ADC) with a -3db point at 6.8 kHz. Next to that, the ADC of the device has a digital sinc5 filter with a cutoff frequency of 0.2 * sample frequency. The Refa of TMSI is connected via two optical cables to the right PC in the lab. But the last meter is a USB cable (under the table). This is according TMSi not a problem, but check: broken link “Thou Shalt Not Allow Thy USB Cabling to Be Cast Loose Upon the Ground, Lest Thee Be Consumed By Fire”. Also standard USB cabling is not intended for use around magnetic fields. It would be better to use EEG equipment that runs on batteries.
Question: could there be problems caused by the long cables of the speakers, for example the picking up and amplifying of 50 Hz electrical noise? Answer: all electrical equipment and cables cause problems. Use the Field Analyzer (NFA 400 of GigaHertz solutions, current location – Sep 2021 - in a black box in the cabinet to the right in the EEG lab) to check the magnetic and electric fields. Put the Data Recorder part of the SAGA close to the subject and seat the subject as far away from cables, sockets and all electrical devices as possible. Advice of TMSi: use electrostatic discharge safe chairs for the subject (with head rest) + use a wooden or at least not-metal bench to put the Data Recorder on. Further use ESD mats everywhere on the floor in the measurement room, see: broken link
The Saga

The SAGA consists of a Docking Station (serial number: 1005200002) and a Data Recorder (sn: 1000200008). To do: add picture and check remarks below.
First install: always docked, then in the first screen of the QRA choose advanced options and then Fiber (Optical connection) => Switch button. If after connecting the fiber to docking and data the green fiber connection light on data is on: no need to dock the data part, but always in the first screen of the QRA choose advanced options and then Fiber (Optical connection) => Switch button.
The 1-bit trigger input (DIGI) of the SAGA can only handle 3.3 volt! The pulse height must be between 2.3V and 3V.
Name in the Signal Viewer: DR Trigger.
(X-axis and y-axis are the internal accelerator)
In the Poly5 the 1-bit trigger is in the STATUS signal: when this becomes 1024 (32 less - bit 5 from the right- than 1056, the default value) a trigger is detected.
Caps
The caps are manufactured by MFI. The caps have integrated passive electrodes. The electrodes are sintered Ag/AgCl electrodes. For electrode positions of the 64 electrodes Refa cap: see Figure 22. This is the 10-10 positioning with some alternations. See: https://www.acns.org/pdf/guidelines/Guideline-5.pdf . The electrode positions of the 64 electrodes SAGA caps is almost identical, only the placement of AF4, AF3 and the GND ground is a bit different. There are also 32 electrodes Refa caps (10-20 positioning with some alternations).
The caps are bought from TSMi, who currently uses Medi Factory as manufacturer. Only the white M cap uses the electrodes from TMSi and the cap from Easy cap. Refa caps can also be used for the SAGA, using the cable adapter.
%todo: add picture.
-
Cap for Refa64, positioning and coding of the electrodes
-
Cap for Mobita32, positioning and coding of the electrodes
Performing EEG measurements
Every new student or new staff needs to be instructed by an experienced researcher before performing an EEG experiment!!
Informing the subject
Tell your subject beforehand how long the experiment will take and sketch the procedure globally. Ask the subject to wash their hair the night before, to use no hair products except shampoo, to get enough sleep and to take a warm sweater – because the lab can be cold. Further inform the subject that no eating or drinking is allowed (except for some water, to be provided by you). After preparing the subject show the effect on the signal caused by eye blinking, swallowing, clamping the teeth and moving in general and instruct the person to sit as relaxed as possible. For an example of a complete checklist see the appendix.
Prepare as much as possible before the subject arrives. All needed supplies, caps, wristband and wires are in labeled boxes in the cabinet to the right when you enter the lab. Please put everything back in the correct place after your measurements (for the cap and wristband: only as soon as they are dry).
Measurement preparation

Needed for skin/head cleaning for EEG preparation (see Figure 23: EEG preparation)
- Disinfectant alcohol for cleaning of the forehead, the mastoid bones, below the eye, next to the eye where the electrodes to measure blinking will be connected. Use this also to clean the scalp (first check with the subject).
- Remark: when the subject used gel or other hair products except shampoo in the hair, the hair needs to be washed first.
- Gauze sponges for applying the alcohol.
- Latex gloves if needed
- Lots of paper + waste bin
- Remark: the use of NuPrep for scrubbing the skin is not recommended. It can be used locally on the exact location where an electrode will be placed, for example the eye electrodes or in case of working with separate electrodes for the mastoids. NuPrep has (low) conducting properties, which makes it unsuitable for overall use because of the risk of electric connections between electrodes.
Needed for making connection between electrodes and scalp
- New (blunt) needle
- Syringe (please don’t re-use so we are sure that we insert clean and fresh gel)
- Abralyt HiCl abrasive electrolyte gel. This contains 10% salt, has a better long term stability and reaches a stable impedance earlier than Abralyt 2000 electrolyte gel. Info of Robert Oostenveld about the electrode-skin interface:
- “In the body current is conducted by ions (Na+, K+, Cl-). In the wires current is conducted by electrons. The skin-electrode interface acts as a half-cell where ions combine with electrons (and vice versa, like in a battery) This causes a constant DC electrode potential of 200mV for AgCl electrodes and gel, more for other materials. Note that ERP effects (~ 1uV) are about ~200.000 times smaller. Impedance changes, sweating, movements, etc. cause small fluctuations in this 200 mV (which are large compared to the ERPs). The gel reduces impedance and stabilizes the half-cell electrode potential compared to dry electrodes.”
- AgCl sticker electrodes in case of working with separate electrodes for the mastoids. But first tests with this did not produce stable results and TMSi advises to use only one type of electrodes in a measurement, because of the DC offset, which is different for different electrodes and changes different for different type of electrodes.
- Further the green ground electrode, a black wrist band to connect it to the ground and the eye track electrodes are needed.
- Add: description of the procedure (make picture) for the application of the EOG electrodes.
- Fitting the cap:
- A good fit of the cap is very important. Use the measurement tape with colors to select the right cap (yellow: small, red: medium, blue: large). The caps are color coded on the velcro that goes under the chin. Measure the circumference of the head and record it – and the following head measurements – in the lab journal.
- First clean the skin and hair/skull with disinfectant alcohol as described above. Nuprep can be used for scrubbing the skin on the mastoids.
- Fit the cap on the head with the cable at the back. Fit tight but check that it is not too tight.
- Measure the distance from the nasion (depressed area between the eyes) to the inion (the most prominent projection of the protuberance which is located at the posterior inferior - lower rear - part of the human skull, for most people you can feel it as a prominent bump). The distance from nasion to FZ should be 0.3 x this distance. Alternatively you can check the distance to Cz, this should be exactly half of the distance of the nasion to the inion.
- Measure the distance from the Tragus of the ear to the ear over the head and check if CZ is exactly in the middle.
The measurement
%todo
Cleaning up after the measurement
%todo
Analysis of the measurement
%todo
Important points concerning the Refa
%todo
The NIRS equipment
%todo
Performing NIRS measurements
%todo
Appendix
%todo
Example of a checklist (ASSR-4DEEG experiment)
%todo
Example of a consent form
%todo